The most important idea in science, and why it’s true
If you’re in the mood to read a great book about physics, there is no author I can recommend more highly than Richard Feynman, the great American physicist and teacher. At the beginning of his famous Lectures on Physics delivered at CalTech (which you can find in Six Easy Pieces), Feynman remarked on the most important idea in science:
If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement would contain the most information in the fewest words? I believe it is the atomic hypothesis (or the atomic fact, or whatever you wish to call it) that all things are made of atoms—little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another.
There are a few reasons why this is such a powerful statement. First of all, it emphasizes how absolutely crucial the idea of the atom is to our understanding of the universe. If you play the game where you keep asking “why?” to every statement someone makes, at the end of the long sequence of why’s you will come to a statement about atoms (or, if you keep going past that, to the Big Bang). This is especially evident if you play the physicists’ version of the “why” game, wherein you keep asking where did the energy come from? Writ large, the things we do are a result of our psychology, and our psychology is a result of our biology, and our biology — the workings of our mind and body — is a result of or so atoms all pushing and pulling on each other in complicated ways. The atom absolutely reigns supreme in our understanding of Nature.
However, knowing that the world is made of atoms doesn’t do you any good if you don’t know how the atoms interact with one another. That’s why Feynman didn’t end his statement at “all things are made of atoms”; it is imperative to our understanding of the world that we know something about how atoms push and pull on each other. Knowing that atoms attract each other when they’re kind of close but repel when they are really close allows us to predict that atoms can pull together to form large structures without collapsing onto the same point.
But why is it true? What is the origin of the attraction and repulsion between atoms? After all, an atom is a neutral object: it has positive and negative charges that completely cancel each other, so why should it attract or repel another atom? The answer is fairly subtle, and surprisingly poorly-known, given that it is fundamental to the “most important idea in science”.
Not a hypothesis any more
The belief that the universe is made of atoms is at least 2500 years old, but until fairly recently it was just that: a belief. During the last 200 years or so the evidence for “atomism” grew increasingly strong, until it really became impossible to imagine an explanation for things that didn’t involve atoms. But it was still sort of a matter of faith, in the sense that you couldn’t actually see an atom.
That is no longer the case. I am sort of amazed to say that I live in an era when people can actually look at individual atoms through high-powered microscopes. Below is a picture of a metal surface taken with an atomic force microscope. The lighter atoms are tin, and the darker ones are silicon.

Of course an atom is not actually a hard ball; what you’re seeing is the electron cloud that surrounds each atom in a mostly-spherical shape. And these days we can do a lot more than just see atoms; we can actually manipulate them individually. The above image is from this paper, where scientists at Osaka University were able to rearrange silicon atoms one at a time to spell out “Si”.
It’s a little surprising to me that the first real picture of an atom wasn’t met with much fanfare. I guess no one was surprised by it, but still, it would have been nice to have some kind of party to celebrate the culmination of 2,500 years of atomism.
Why atoms attract
Roughly speaking, an atom is made of two parts: the positive nucleus and the negative electron cloud. And, most of the time, these parts exactly cancel each other so that the atom is neutral. Here is a rough picture of one:

an (ugly) atom, with its positive nucleus in the middle and a negative electron cloud surrounding it
When the electron cloud is exactly centered around the nucleus, there is no electric field outside the atom. Thus, the atom should not push or pull on anything else. But it can happen that the electron cloud gets shifted to the side a little bit, so that it is no longer centered around the nucleus. When that happens, electric forces pull on both the nucleus and the electron cloud to bring them back together.

The result is a kind of “atomic springiness”: when the nucleus gets separated a little from the center of the electron cloud, electric forces arise that pull the two back together. And so the electron cloud can oscillate around the nucleus if it is disturbed a little bit.
As it turns out, the electron cloud is always oscillating around the nucleus. This is a result of quantum mechanics, which says that any object in a trap (e.g. the electron cloud stuck in the potential well of the nucleus) will bounce around with a characteristic frequency.
So the electron cloud of an atom is not always centered around the nucleus, but rather is fluctuating back and forth. And when the nucleus and the electron cloud are not centered, there is a resulting dipole moment, which means that there is an electric field surrounding the atom. This field pulls on other nearby atoms.
Imagine, for a moment, two isolated atoms, each of which has an electron cloud oscillating around its nucleus. At first the two atoms may have completely independent oscillations, but after a while the oscillations of one atom start to affect the other atom. The two atoms start to oscillate together (in phase), so that the positive side of one atom is always facing the negative side of the other, and vice-versa. As a result, the atoms begin to pull toward each other. I imagine it something like this:
In physics language, we would say that the dipole field of one atom induces a dipole in the other atom, and we call this the Van der Waals force. It’s pretty weak compared to the attraction between individual charges and it has a short range. Of course, it’s still strong enough to allow geckos to climb on walls.
Why atoms repel
We’ve seen that atoms pull themselves together, but why should they stop? Why not just keep pulling until the two atoms sit right on top of each other?
The answer, once again, comes down to quantum mechanics. This time it involves the Pauli Exclusion principle, which says that two electrons cannot occupy the same space and have the same energy. If we brought our two atoms extremely close together, then their electrons would occupy the same volume. This cannot happen unless some of the electrons move to higher energy levels. So pushing the atoms together requires a lot of energy: enough to move about half the electrons to a higher energy state. And whenever moving in a particular direction costs energy, a force arises that pushes in the opposite direction.
Here is a schematic depiction of the process of pushing two atoms together:
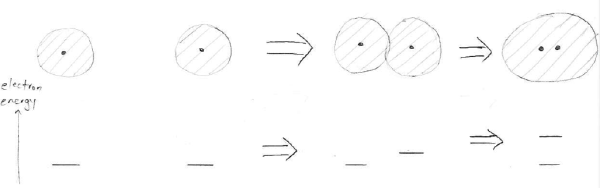
The top row shows the atoms being pushed together and the bottom row (the little horizontal lines) shows the energy levels occupied by the electrons. Once the electron clouds of the two atoms start to overlap, their electrons can no longer have the same energy, but must go to a higher energy state. So the atoms would “prefer” to remain apart, at least enough that their electron clouds don’t overlap strongly.
There are lots of details that I’m omitting here. The strength of the repulsion between atoms depends a lot on the structure of their electron clouds. This is the rich and wonderful playground of chemistry. The hydrogen atom, for example, has essentially no energy cost associated with being pushed into another hydrogen atom because there are two equivalent energy states for each of the electrons to occupy. A helium atom, on the other hand, repels other heliums fairly strongly because there is a big difference between the energy level for the first two electrons and the energy level for the second two. That’s why hydrogen atoms naturally form the molecule but helium atoms remain separated.
Putting it together: Lennard-Jones
The attraction and repulsion of atoms is often put together to form something called the “Lennard-Jones” potential, which looks something like this:
 On the attractive side, the force goes to zero like
On the attractive side, the force goes to zero like , where r is the distance between atoms. This law is fairly easy to derive by thinking about “one dipole inducing another”. The repulsive side has the force going to infinity like
. I don’t know of any derivation of this fairly strange result, and as far as I know it is a purely experimental fact.

Good post…..takes me back to my Physics degree.!!
You are right about the 1/r^13 force – check out page 57-58 of Kittel ‘s “Intro to Solid State” (mine is 8th ed) – there he states that it is an empirical fit. An exponential fit, A*exp(-R/R0), is another functional form that is often used to model the Pauli repulsion term.
Thanks for the explaination. I believe we see the exact same pull/repell with people. There is the need for connection…and we all need space to survive!
The question of whether something like the Lennard-Jones potential can be used to describe social interactions is a pretty good one. We really do tend to have a “long-range attraction” and “short-range repulsion” in a lot of situations.
Here’s one that comes to mind:
A scientist has a significant incentive to align his research interests with those of other scientists (“align his polarization”). That way he can become part of a community, have his work better understood and appreciated, and ultimately be better funded. So his research is naturally “attracted” to some community of other scientists, and as a consequence becomes similar.
However, there is a very strong disincentive if he gets too close. If a scientist’s research is exactly the same as someone else’s, then he will have to compete for funding and risks being considered irrelevant or dispensable. So at the moment that the specific research problems of two scientists “overlap” strongly, there is a “social force” of repulsion.
I know that there are people in physics who research social networks and opinion formation, but I don’t know whether anyone uses this kind of language to describe them.
Cool post, but I disagree with you on one major point. I don’t think this idea is the most important idea in science. I think the most important idea in science is the scientific method. Without it, our understanding of the atom and its forces would never have been found…but more importantly, our understanding would never progress beyond what it currently is.
I agree with Danny that the scientific method is absolutely pivotal. However, the scientific method itself is not a scientific fact per se, but is rather a meta-scientific or philosophical fact. That is, one cannot prove the scientific method by scientific means, because the scientific method is a first or starting principle. You can’t prove the scientific method by using the scientific method any more than you can prove the law of noncontradiction in logic to prove itself.
You edit your posts a lot — I believe you removed a discussion of whether this is a “purely experimental fact,” and you added caveats to your other post about humans living to 130. After a post has been up for a while and a lot of people have seen it, I think most bloggers put an “Updated” note in the post saying they’ve made changes. At least it feels weird to come back to a post and have it be all different.
This particular post hasn’t been edited — the discussion your referencing is in the last paragraph and in the comments above. But you’re right, I’ll be more careful about that in the future.
Reading this put me in mind of CR Woese’s article, A New Biology for a New Century, Microbiology and Molecular Biology Reviews, June 2004, p. 173-186, Vol. 68, No. 2, where he describes “fundametalist reductionism,” and why it’s bad for biology.
I am not sure if 20th century physics did reject this view. Feynman’s quote makes me think not. And of course it is hard to call “metaphysical” something you can take a picture of! But physics sure is getting weird, what with dark energy and dark matter. Is the atomic hypothesis going to work for these? I would guess, probably yes. What do others think?
I’m not sure there’s such a conflict between the two ways of thinking. There doesn’t seem to be anything inconsistent between saying “a human is just a large collection of atoms”, and “a human exhibits an enormous array of complex, emergent behaviors.”
As I feel it, the current attitude in physics is very much a materialistic one. Everything is made of physical objects which obey deterministic (or at least probabilistic laws). But any system of many interacting objects can exhibit complicated emergent behavior. In essence, we have to allow both statements to be true.
And you’re right that the atomic hypothesis no longer science’s most fundamental statement. There are more delicate are more nuanced pictures of matter these days: atoms are made of electrons, protons and neutrons, of which the latter two are made of quarks. And there are other kinds of particles that don’t appear in atoms. But thinking of the world as made of indivisible “building blocks” called atoms still gets you a long way.
“But thinking of the world as made of indivisible ‘building blocks’ called atoms still gets you a long way.” Yes, it appears to have brought physics all the way to the Standard Model. (A name I know, which means I know nothing. HT: Prof. Feynman.)
I certainly am a materialist, and assume Woese is, too. But emergent behavior in physical systems might require a different way of thinking about matter and energy. But it will still be “just” matter and energy. Woese posits that biology will lead the way, and that seems possible. PLoS Computational Biology is full of articles by physicists.
I like this blog. Thanks. Was led here by Kenneth Anderson at the Volokh Conspiracy.
I don’t believe the question is whether there is a “conflict” between fundamentalist explanations of nature and emergent behavior. If there were a conflict I suppose we wouldn’t see emergent behavior. The problem seems to me to be that the phenomenon of emergence appears to render the fundamentalist explanation inadequate, in principle. We used to think that anything could be explained by reduction, if we just knew enough. Now we are somewhat at a loss. And the”complexity” of emergent behavior is not at all what makes it mystifying.
Seeing atoms one by one, and the ability to move an individual atom, is indeed cool. It is not, however, all that new. Dr. Mueller of Pann State built a field ion microscope back in the sixties, or maybe even the fifties. I used one at the Air Force Materials Lab in 1962 to study the properties of iron whiskers. By diddling with the potential between the target whisker and the anode you could not only see the planes of atoms in the iron crystal, you could peel the layers off one by one.
The field ion microscope was Dr. Mueller’s second try, the first being the field electron microscope. Its resolution was not as good because the electron wavelengths were too large with respect to the lattice spacing. Hydrogen ions worked much better, enabling resolution down to an angstrom or so.
Before I worked for the Air Force, I worked with Dr. Koehler at the U of Ill. As a lab assistant, I got to plot the closed-shell repulsive potential, as revealed in experiments involving extremely high-velocity collisions between various chunks of metal. As I recall, the guns used were getting their projectiles up to about mach 6
The existence of atoms was demonstrated by Ben Franklin (though he did not draw the conclusion). He poured a teaspoon of oil on a pond and discovered it would stop spreading after it covered a finite area (roughly an acre). The oil-covered surface is distinguishable because the oil suppresses surface capillary waves, smoothing the surface. From that result we can infer the size of oil molecules and the existence of discrete indivisible (without changing their properties) molecules or atoms.
The 7th/13th power force law is called the Lennard-Jones 6-12 potential (expresses as potential energy rather than force, the exponents are 6 and 12). The 6 is the van der Waals attraction. The 12th power is not an analytic result, but simply chosen for algebraic convenience and the need for a stiff “hard core” repulsion. Sometimes a “double exponential” potential is used, with a repulsive core with large coefficient and a short e-folding length that dominates at small distances, and an attractive longer-range potential. Of course, that does not match the van der Waals potential at the longest ranges, but it is a good fit near the turning point.
Yes, at this moment I’m not sure if the author is saying that the above picture of atoms (as spherical balls lined up with each other) was made using Electron microscope tunneling or otherwise. And, if so, it’s my understanding that this process produces only an approximation of the atom’s appearance. Using mathematical formulas to fill in the gaps.
So is it really correct to say that it’s outer appearance is spherical? Why not oblong? (Since, as I understand it, some orbitals aren’t spherical at all?) And also, why should the outer boundaries be so sharp and distinct like a billiard ball? Again, why not fuzzy and indistinct?
I can’t help but think how similar this is to astronomy, i.e. Planets and gravity… Wow, such interesting stuff…
Yes, I have seen pictures of atoms like the one above. And have also made comments to the effect that the spherical shape of the atom as shown by the electron microscope can only be an approximation. Since electron microscopes don’t take pictures using light. But gather other kinds of information.
But now I see (by what was said above) that if the electron is always oscillating about the nucleus then an electron cloud would naturally manifest itself. So that the spherical shape of the atom isn’t some arbitrary design arising from an artist’s imagination As in Neils Bohr’s planetary conception.
However, another next question that still lingers is how an electron can manifest itself both as a wave and a point particle at the same time?